Convection
Convection is heat transfer from a solid to a liquid or vise-versa. It is driven by diffusion.
It is given by the following equation:
Where
Fluid Flow
Chapter 6 reviews convection in depth, fluid flow concepts are heavily involved in this chapter.
Nodal Network
Nodal network is an approximation method to analyze heat transfer in two dimensions (3D will be covered later). They are coved in section 4.4 of the textbook.
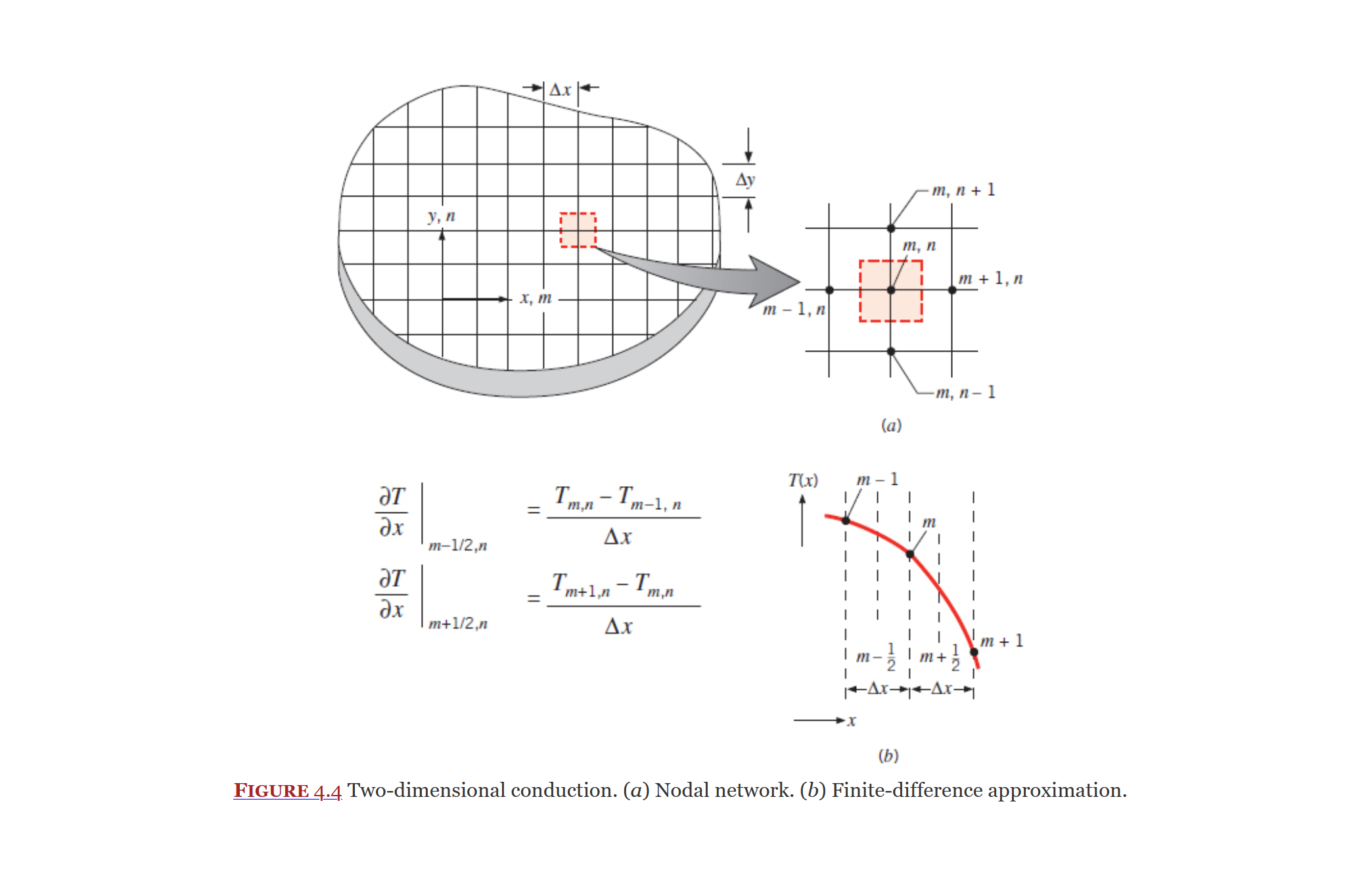
The item you want to analyze is sectioned into nodes (an interior node is shown below), from there the heat transfer rate into and out of the node is balanced.
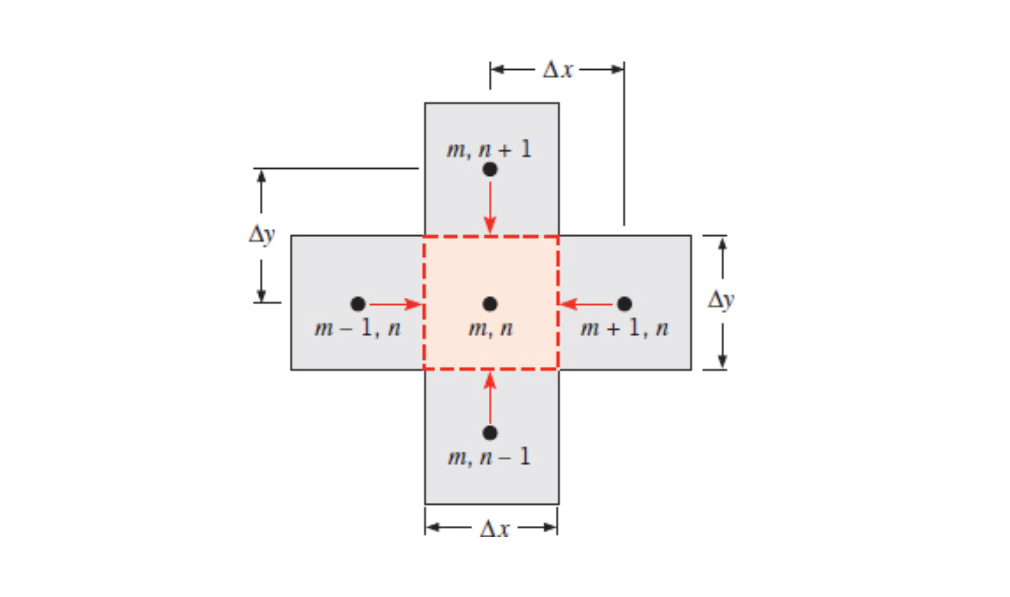
All flows into/out of the node are drawn going into the node (negative heat transfer rates are used to show flows out of the node). From here the heat rate balance can be reduced significantly. See 5A - Class 2 - Finite Difference for an example derivation.
Table of Nodal Finite Difference Equations contains reduced equations for common 2D node conditions.
Finite Difference Analysis
Finite Difference Analysis, also known as finite-element or boundary-element, is a method of analysis that use finite values rather than the continuous ones of other methods.
It is an approximation rather than an exact result.
This method makes use of a nodal network to break a solid up into finite elements.
See the following for examples and more information:
Energy and Heat Flow
Heat Flows from hot to cold, we call this heat-transfer-rate.
Heat can flow via 3 methods.
Measurements of Heat
Regardless of how the heat is bring transferred we have to be able to measure it.
Conduction with Heat Generation
This section covers how to analyze conduction problems that include a heat generating solid. It is coved in Section 3.5 of the textbook.
Equation 3.44:
Yields the general solution (Equation 3.45):
Equation 3.46:
Where
You cannot use thermal circuit resistance to find a temperature within a heat generating solid.
Volumetric Heat Generation
For materials that generate heat (like nuclear fuel) this is used to measure the amount of heat they generate.
As more mass is added, more heat is generated. Thus this is a function of volume of the heat-generating material.
If you know the width of the heat generating solid (
Conduction
Conduction is the transfer of heat through collisions between atoms/molecules and the diffusion of atoms/molecules of high energy. It is mainly defined by Fourier's Law.
It can occur inside a fluid or a solid. See ME3304 S24 Lecture Note (1) Introduction for more info.
One Dimensional
For one-dimensional (x direction only), steady-state, heat diffusion we find that depends on the temperature difference across the solid, the area perpendicular to the flow -
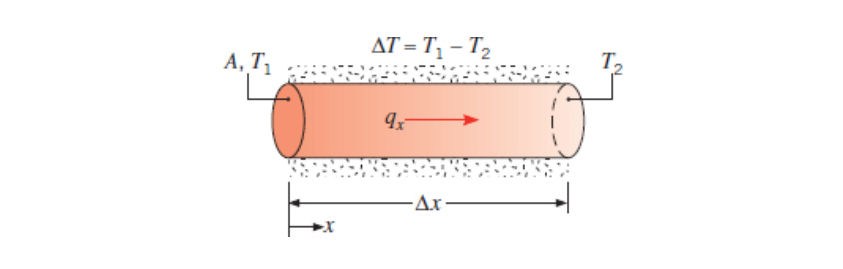
Thus we can calculate the heat transfer rate as the following:
Again,
We call
We can convert this to heat flux by moving the area over.
Two Dimensional
With Heat Generation
Semi Infinite Solid
A method that considers space and time.
- Dr. Paul
In contrast to lumped capacitance method which only works on problems that are a function of time, semi-infinite solid method works on problems that are a function of both location and time.
Semi-Infinite Solid is an analysis method that uses a solid that is of infinite size in all but one direction. It is used to analyze temperature of an object over time.
This method yields an analytical solution rather than an approximation, although you are still making an approximation in the fact that infinite solids do not exist. Unlike the lumped capacitance method it can be used where there is a temperature gradient across the solid we are analyzing (the biot number is not very small).
Below are example transient temperature distribution for constant surface temperature (1), constant surface heat flux (2), and surface convection (3).
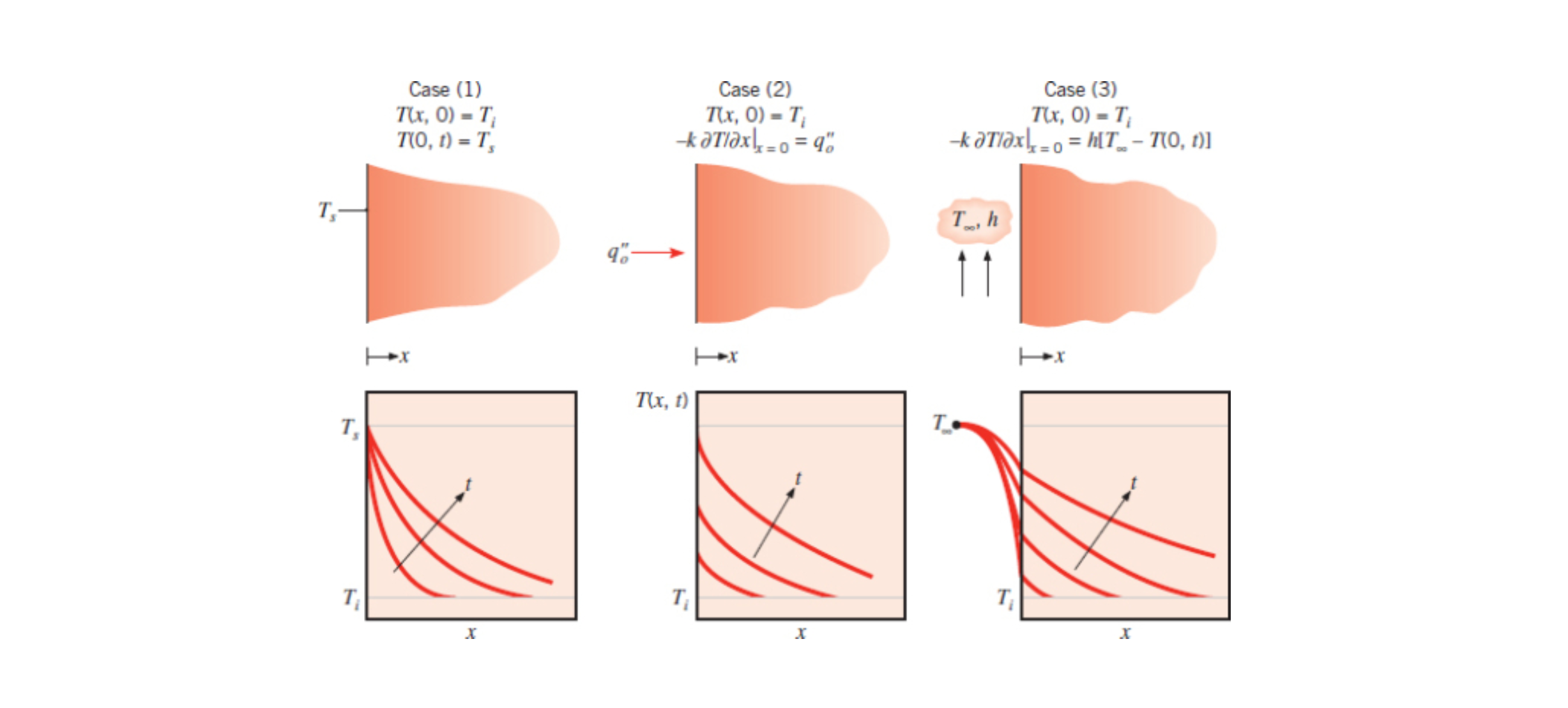
See Section 5.7, 6E - Class 2, 7A - 2nd Class, and 7C - 2nd Class.
Temperature distribution
The derivation for the following equations (and what the error function is and where it came from) can be found in 7A - 2nd Class.
Equation 5.60:
Where
Case 1 Constant Surface Temperature (Eq. 5.61):
Case 2 Constant Surface Heat Flux (Eq. 5.62):
Case 3 Surface Convection (Eq. 5.63):
Where
Python
Equation 5.63 (Case 3 above):
def caseThree(T, Ti, Too, x, alpha, t, h, k):
coeffOne = erfc(x/(2*sqrt(alpha * t)))
coeffTwo = exp(((h*x)/k) + ((h**2 * alpha*t)/k**2))
coeffThr = erfc((x/2*sqrt(alpha*t)) + ((h*sqrt(alpha*t))/k))
coeffFou = (T-Ti)/(Too-Ti)
return coeffOne - coeffTwo * coeffThr - coeffFou
Heat Flux
Heat flux is heat transfer though a surface. It is perpendicular to the surface it is traveling through.
You can relate heat flux to heat transfer rate using the area of the surface (
When working with one-dimensional convection and under steady-state conditions we can define heat flux as the following.
Heat Transfer Rate
Heat transfer rate is the rate of energy transferred between objects via heat. It has units of watts.
When working with thermal circuits heat transfer rate is considered to be current.
Thermal Diffusivity
Thermal Diffusivity is the diffusion coefficient for heat. Just like there is a diffusion coefficient for momentum, etc.
- Dr. Paul
Thermal diffusivity,
Page 58 of the textbook (Section 2.2.2).
Fourier Number
The Fourier Number is dimensionless time and is used with the biot number to characterize transient conduction problems. It is defined in equation 5.12 (Section 5.2) as the following:
Where
Lumped Capacitance Method
Lumped Capacitance really means that heat transfer is a function of time only.
- Dr. Paul
Lumped Capacitance Method is a continuation of the ideas laid down in thermal circuits. As the name suggests we now have a thermal capacitance,
This method is an approximation and it only works is there is no temperature gradient across the object. This can be quantified by checking to make sure the biot number is much smaller than 1. See ME3304 S24 Lecture Note (2) Conduction.
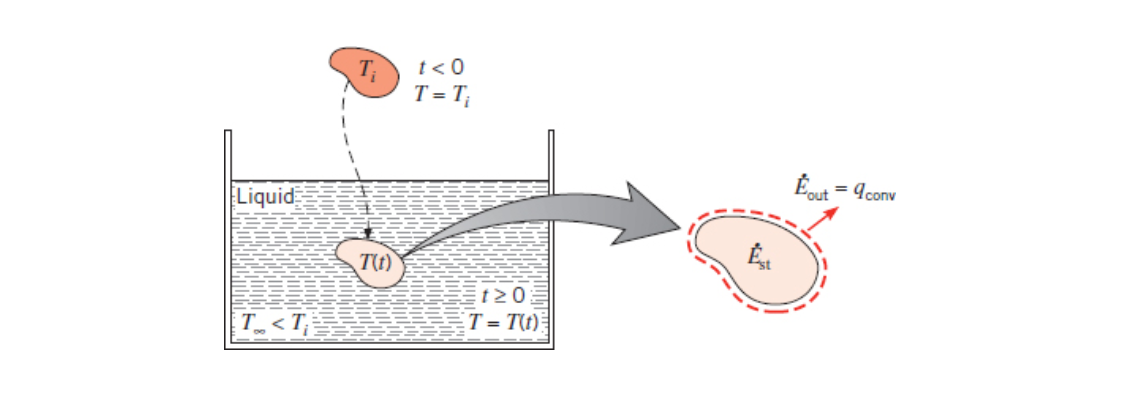
Given a solid of a high temperature that is in an environment that rapidly changes temperature (for example heat treating metal) we imagine that the solid is a capacitor (or battery). As soon as our solid changes environment it will start to discharge its energy as heat.
Equation 5.4 (temperature difference):
Equation 5.5:
Where
Equation 5.6:
See also ME3304 S24 Lecture Note (2) Conduction.
Where
Relating to thermocouple somehow.
An example of a problem using this method can be found in ME3304 Conduction Previous Test Solution.
Allows you to write energy-stored as the following?
Heat Diffusion Equation
Equation 2.19 (below) is the general form of the heat diffusion equation in cartesian coordinates.
Where
Cylindrical Coordinates (Eq. 2.26 - Pg. 65)::
Spherical Coordinates (Eq. 2.29 - Pg. 67):
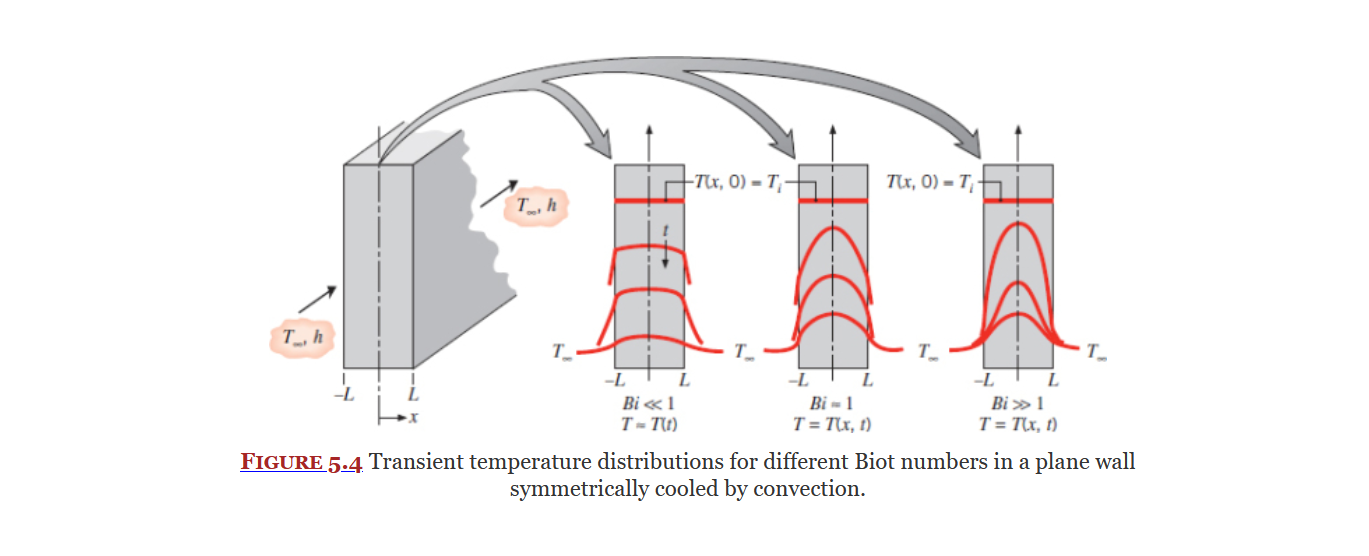
Biot Number
Biot Number (Eq. 5.9) is a dimensionless parameter. It is a measure of how dramatic the temperature difference across a solid is in comparison to the temperature difference across the boundary undergoing convection.
Where
Section 5.2 goes says the following:
Biot number provides a measure of the temperature drop in the solid relative to the temperature difference between the solid's surface and the fluid.
From Equation 5.9, it is also evident that the Biot number may be interpreted as a ratio of thermal resistances.
In particular, if Bi ≪ 1, the resistance to conduction within the solid is much less than the resistance to convection across the fluid boundary layer.
It is used in the lumped capacitance method as well as to tell if the fin approximation can be used... I think, idk.
Fourier's Law
Fourier's Law essentially states that heat flux is always normal to a cross sectional area of constant temperature.
Where
See ME3304 S24 Lecture Note (1) Introduction for more information.
Energy Balance
Heat Transfer lives as a subcategory of thermodynamics and is based off its ideas. Energy balance is one of thermodynamics' defining characteristics and is true for heat transfer.
Starting with the a basic energy balance about a control volume we have:
Where
We can develop this further by accounting for energy stored within a control-volume and energy generated by a control volume using the following equation:
See Section 1.3 of the textbook for more information.
This concept is applied to systems using conduction by the heat diffusion equation.
Specific Heat
Specific heat is a measure of how much energy is required to raise a mass by one degree. See below for units.
Specific Heat has units of joules per kilogram kelvin in the metric system. Water has a specific heat of 4181 J/kgK. Therefor 4181 Joules are required to heat up one kilogram of water by one degree Kelvin.
Thermal Capacitance
Built off the idea laid down in thermal circuits, Thermal Capacitance is essentially the idea of electrical capacitance applied to heat. That is to say it is a measure of a materials ability to store heat. It is the driving idea lumped capacitance method.
Where
Thermal capacitance is covered in section 5.1 of the textbook (page 192).
Thermal Circuits
In many cases heat flow can be analyzed using thermal 'circuits'. When using this approach heat transfer rate becomes current, temperature difference becomes voltage, and resistance is the material's resistance to the heat flow.
Thermal circuits are covered in chapter 3.
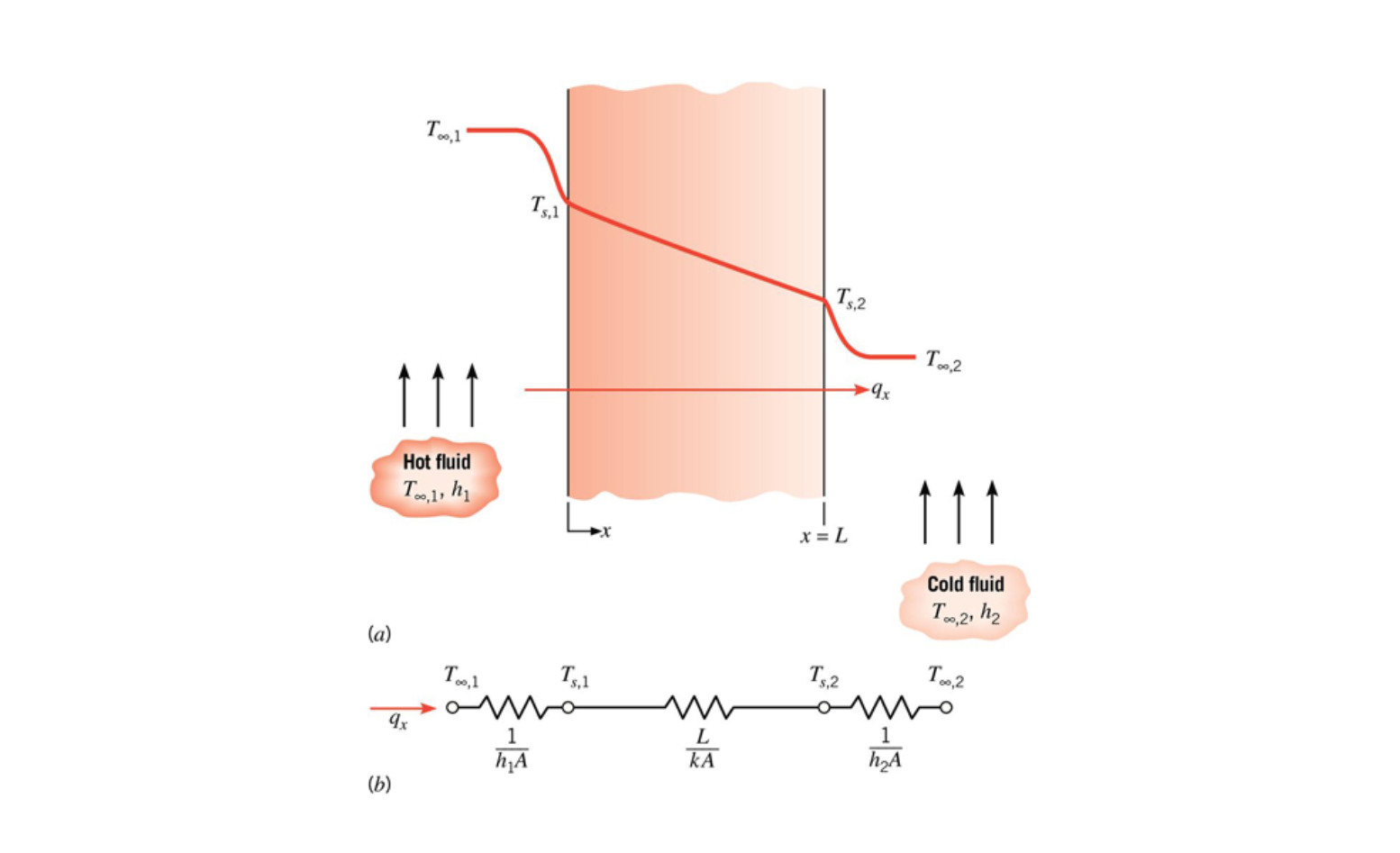
Resistance for conduction:
Where
Resistance for convection:
Where
Contact Resistance
Section 3.1.4
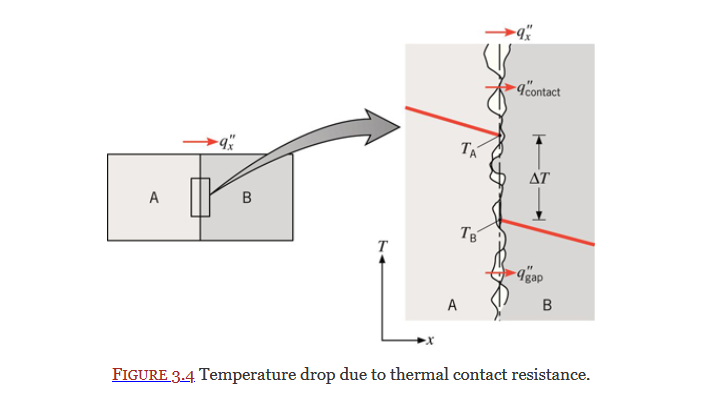
Equation 3.20:
Thermal Capacitance
Thermal capacitance is an idea that builds upon the foundations laid down in thermal circuits. It is explained in its own page.
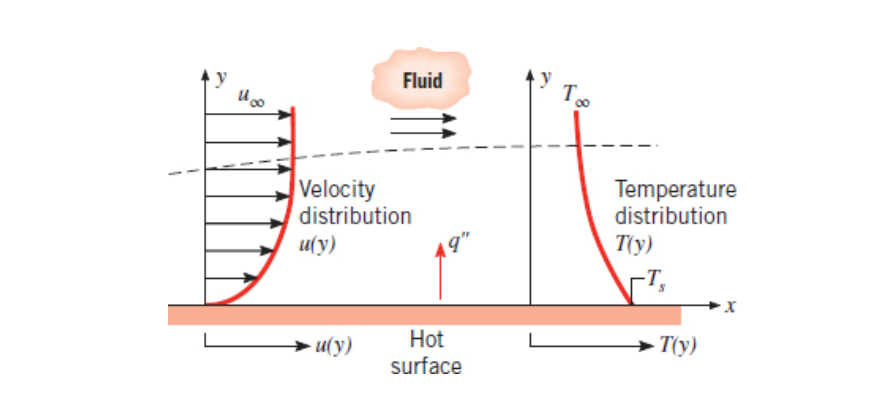
Thermal Boundary Layer
The thermal boundary layer is a fluid boundary layer that is measures the temperature of a fluid as it flows over a surface. The concentration boundary layer is essentially the same thing but it measures mass instead.
Heavily related to velocity boundary layer.
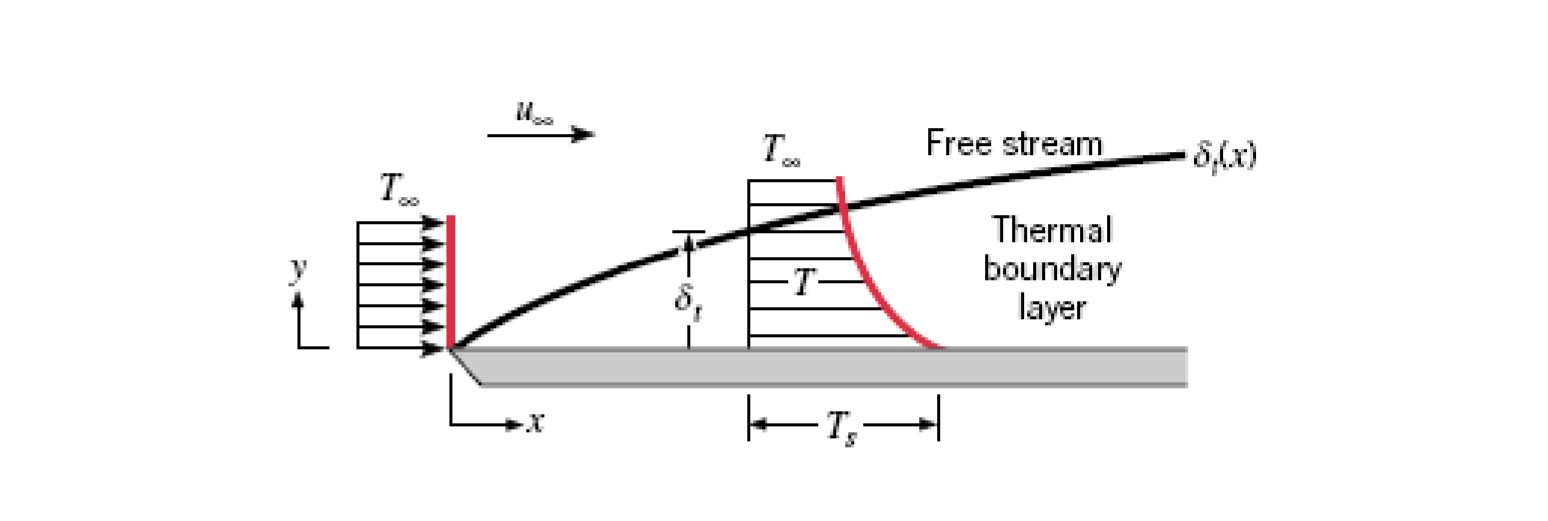
The thermal boundary layer thickness,
Heat flux given by the following:
Using this we can find an expression for the convection heat transfer coefficient. If
Then plugging this into our equation for heat flux we get:
See ME3304 S24 Lecture Note (3) Convection for more info.
Equation 6.14:
The derivation of Equation 6.14 is shown in 9A - 2nd Class.
Internal Flow
Chapter 8
Internal fluid flow is fluid flow that occurs within a pipe or other container.
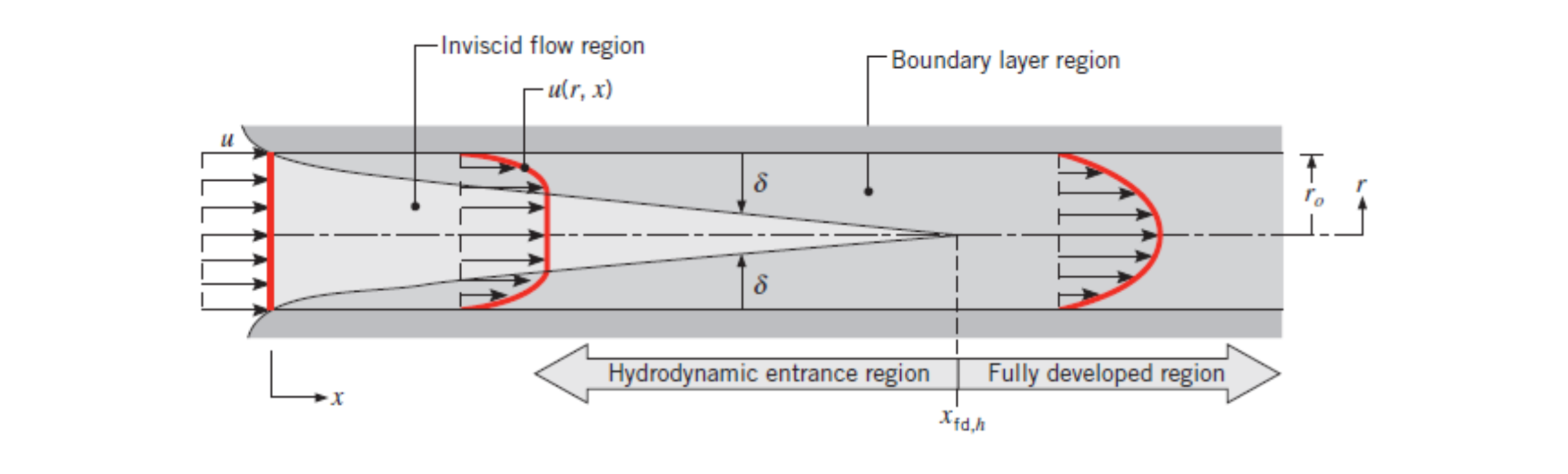
If the flow is laminar (ie. in the fully developed region) the velocity profile is parabolic. See entry-region for how to calculate the distance at which the fully developed region starts.
When dealing with turbulent flow it becomes extremely difficult to find the temperature gradient so instead we use the mean temperature (or mixed bulk temperature).
For internal flow the characteristic length is known has hydraulic diameter.
Mass Flow Rate (Equation 8.5):
Where
See Chapter 8 and ME3304 S24 Lecture Note (3) Convection for more info.
Energy Balance
Section 8.3
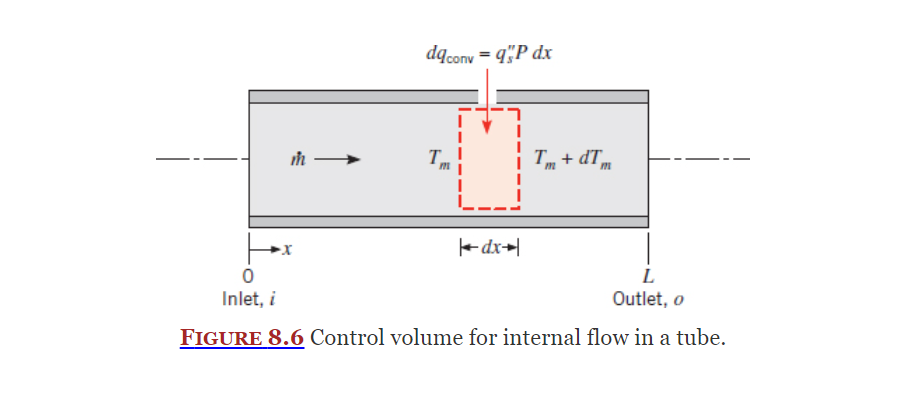
As the flow in completely enclosed, an energy balance can be used to find the mean temperature.
Equation (8.34):
See 7C - 1st Class.
Constant Surface Heat Flux
Covered in Section 8.3.2, analyzing internal flow with constant heat flux is greatly simplified as heat flux becomes independent of location. Equation 8.38 gives convection heat transfer rate:
As shown in 7C - 1st Class and section 8.3.2 we can derive an expression for temperature as a function of location (Equation 8.49):
Idk what Figure 8.7 is, its just "very important" or smth
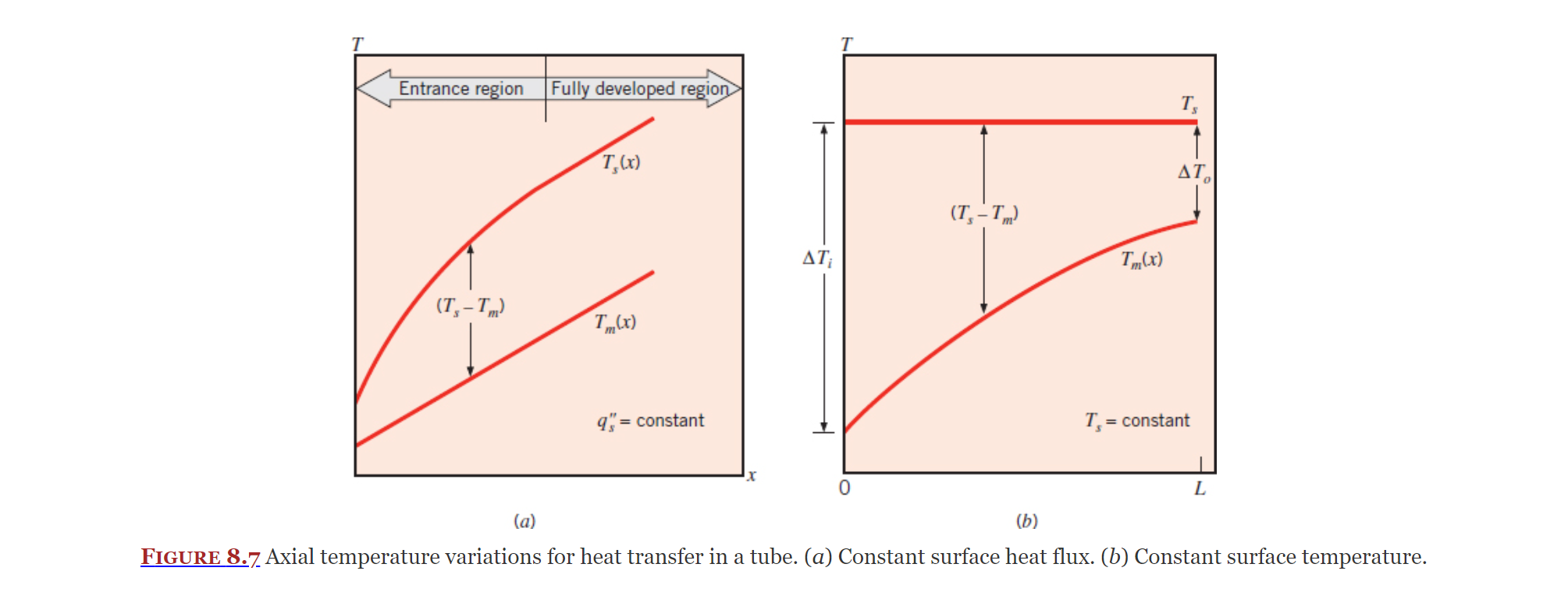
Constant Surface Temperature
Covered in Section 8.3.3, another simplification we can take advantage of is when there is a constant surface temperature.
Equation 8.41b:
Equation 8.42:
Where
Equation 8.45:
See 7C - 1st Class.
Flow around a Sphere
Section 7.5
Stokes' Law (Equation 7.55):
Where
Equation 7.56:
Where
Equation 7.57:
Impinging Jets
Section 7.7
Laminar Flow
A fluid-flow concept.
Laminar Boundary Layer
In contrast to turbulent-boundary-layer.
You can find the point at which flow becomes turbulent using the critical reynolds number.
See ME3304 S24 Lecture Note (3) Convection for more info.
Critical Reynolds Number
Covered in Section 6.3.1, the critical Reynold's Number is the Reynold's number at which the boundary layer flow transitions from laminar to turbulent.
The Critical Reynold's Number is defined in equation 6.24:
Where
See 9A - 2nd Class for more information.
Reynolds Number
A dimensionless parameter that is commonly used to characterize fluid flow, generally turbulent flow.
Where
The Critical Reynold's Number is used to find the point in a boundary layer at which the flow transitions from laminar to turbulent.
Dimensional Analysis
A fluid-flow concept, relies heavily on the use of dimensionless parameters.
See:
Dimensionless Parameters
Parameters that have no unit of measurement, commonly used in dimensional analysis for analyzing fluid flow.
All dimensionless parameters are just ratios of one thing to another.
- Dr. Paul (paraphrased)
In heat transfer many dimensionless parameters use the star notation.
Table of Heat Transfer Dimensionless Parameters
Table 6.2 is a list of dimensionless parameters applicable to heat transfer.
| Group | Definition | Interpretation |
|---|---|---|
| Biot Number (Bi) | Ratio of the internal thermal resistance of a solid to the boundary layer thermal resistance | |
| Mass transfer Biot number (Bim) | Ratio of the internal species transfer resistance to the boundary layer species transfer resistance | |
| Bond number (Bo) | Ratio of gravitational and surface tension forces | |
| Coefficient of friction (Cf) | Dimensionless surface shear stress | |
| Eckert number (Ec) | Kinetic energy of the flow relative to the boundary layer enthalpy difference | |
| Fourier number (Fo) | Ratio of the heat conduction rate to the rate of thermal energy storage in a solid. Dimensionless time | |
| Mass transfer Fourier number (Fom) | Ratio of the species diffusion rate to the rate of species storage. Dimensionless time | |
| Friction factor (f) | Dimensionless pressure drop for internal flow | |
| Grashof number (GrL) | Measure of the ratio of buoyancy forces to viscous forces | |
| Colburn j factor (jH) | St Pr2/3 |
Dimensionless heat transfer coefficient |
| Colburn j factor (jm) | Stm Sc2/3 |
Dimensionless mass transfer coefficient |
| Jakob number (Ja) | Ratio of sensible to latent energy absorbed during liquid–vapor phase change | |
| Lewis number (Le) | Ratio of the thermal and mass diffusivities | |
| Mach number (Ma) | Ratio of velocity to speed of sound | |
| Nusselt number (NuL) | Ratio of convection to pure conduction heat transfer | |
| Peclet number (PeL) | Ratio of advection to conduction heat transfer rates | |
| Prandtl number (Pr) | Ratio of the momentum and thermal diffusivities | |
| Reynolds number (ReL) | Ratio of the inertia and viscous forces | |
| Schmidt number (Sc) | Ratio of the momentum and mass diffusivities | |
| Sherwood Number (ShL) | Ratio of convection to pure diffusion mass transfer | |
| Stanton number (St) | Modified Nusselt number | |
| Mass Transfer Stanton number (Stm) | Modified Sherwood number | |
| Weber number (We) | Ratio of inertia to surface tension forces |
Fully Developed Region
The fully developed region of an internal flow is the point at the boundary-layer merges with itself. See photo below:
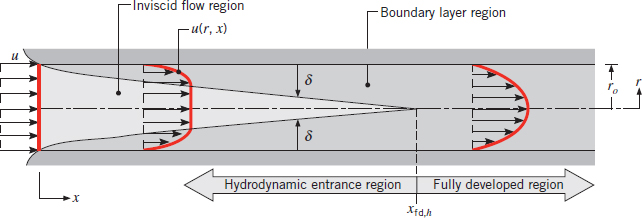
Section 8.1.1
Flow in Noncircular Tubes
Section 8.6
Concentration Boundary Layer
The concentration boundary layer is like thermal boundary layer but if the temperature was instead mass.
Essentially measures density?
Reynolds Analogy
The following equation (Equation 6.69) is known as the Reynolds Analogy. It allows other parameters to be found if the velocity is known and vise versa. It consists of dimensionless parameters.
Where
This analogy is further expanded by the Modified Reynolds analogy (Eq. 6.70):
And the Chilton-Colburn analogy:
See Section 6.7.3 (page 298) and ME3304 S24 Lecture Note (3) Convection.
The following is from lecture 7A, not sure what it means or if its true
Flat Plate in Parallel Flow
Section 7.2 covers a the convection associated with an isothermal flat plate exposed to flow on one side as shown in the below image.
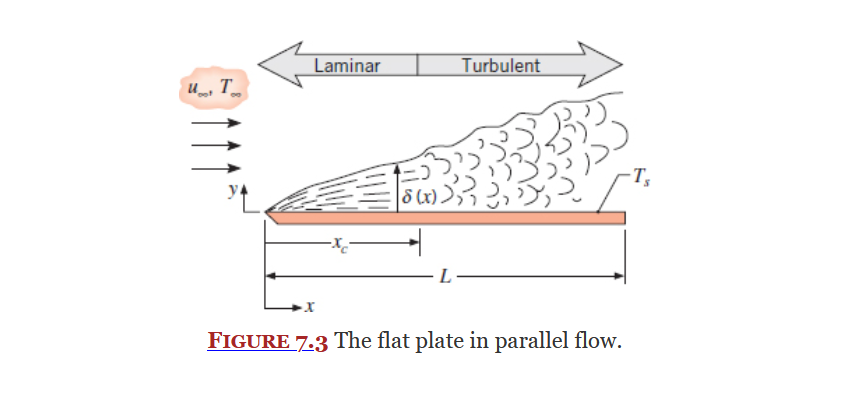
See boundary layers, specifically thermal boundary layers.
Using the similarity transformation on the boundary layer equations, the following equations for continuity, momentum, and energy can be found:
Continuity (Eq. 7.4):
Momentum (Eq. 7.5):
Where
Energy (Eq. 7.6):
Where
See ME3304 S24 Lecture Note (3) Convection and Example 7.1 for more information.
Laminar Flow over an Isothermal Plate
Section 7.2.1
Equation 7.29:
Where
Equation 7.30 (temperature):
Where
Equation 7.31 (mass):
Where
Turbulent Flow over an Isothermal Plate
Section 7.2.2
Equation 7.34:
Where
Velocity boundary layer thickness (Equation 7.35):
Equation 7.36 (temperature):
Where
Equation 7.37 (mass):
Where
Mixed Boundary Layer Conditions
Section 7.2.3
Equation 7.38:
Where
Equation 7.40:
Where
Equation 7.41:
Where
Other conditions
Section 7.2.4 covers a unheated starting length
Section 7.2.5 covers Flat Plates with Constant Heat Flux
Laminar Flow in Circular Tubes
Section 8.4 covers laminar flow in circular tubes (internal-flow).
In the fully developed region
Constant Heat Flux (Equation 8.53):
Constant Surface Temperature (Equation 8.55):
The derivation of equations 8.55 and 8.53 can be found in 11A - 2nd Class.
In the entry region
Equation 8.56:
Equation 8.57:
Equation 8.58:
Entry Region
The entry region is the region before the fully developed region in an internal flow.

Turbulent Flow in Circular Tubes
Section 8.5
Fully Developed Flow
The Dittus-Boelter equation (Equation 8.60) is valid for small temperature differences:
Where
The derivation for the Dittus-Boelter Correlation can be found in 11A - 2nd Class.
Large Temperature Differences
Equation 8.61:
Smooth tubes
Equation 8.62 is more accurate than 8.61 but only applicable to smooth tubes:
Velocity Boundary Layer
Heavily related to thermal boundary layer.
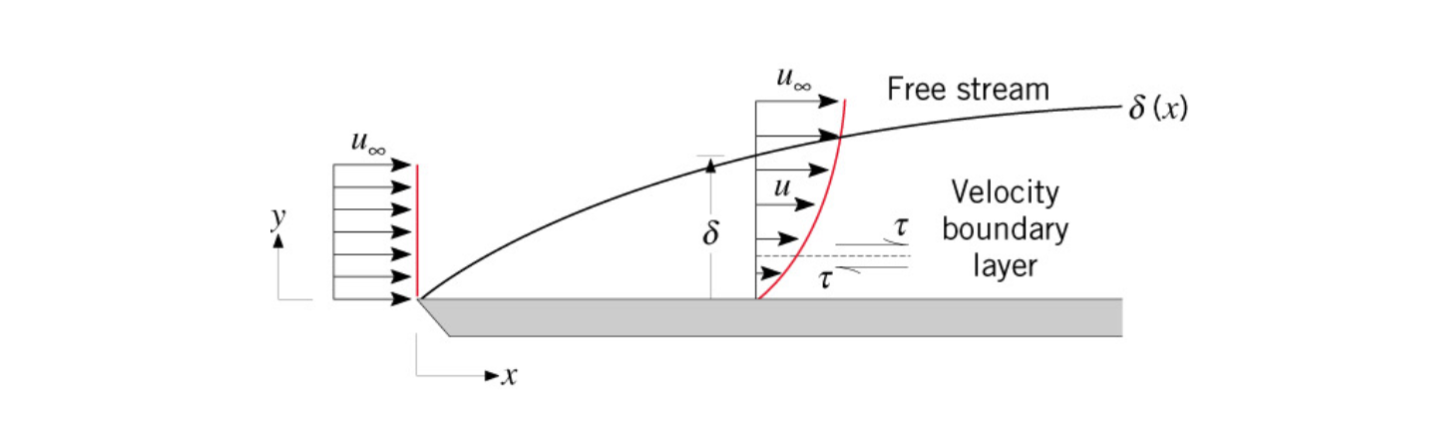
Surface frictional drag may be found using the friction coefficient. (Section 6.1.1)
The edge of the boundary layer is where the fluid it traveling 99% of the free stream velocity.
Turbulent Flow
A fluid flow concept.
Everything is more in turbulent, more heat transfer, more drag, etc.
- Dr. Paul
Turbulent Boundary Layer
In contrast to laminar-boundary-layer.
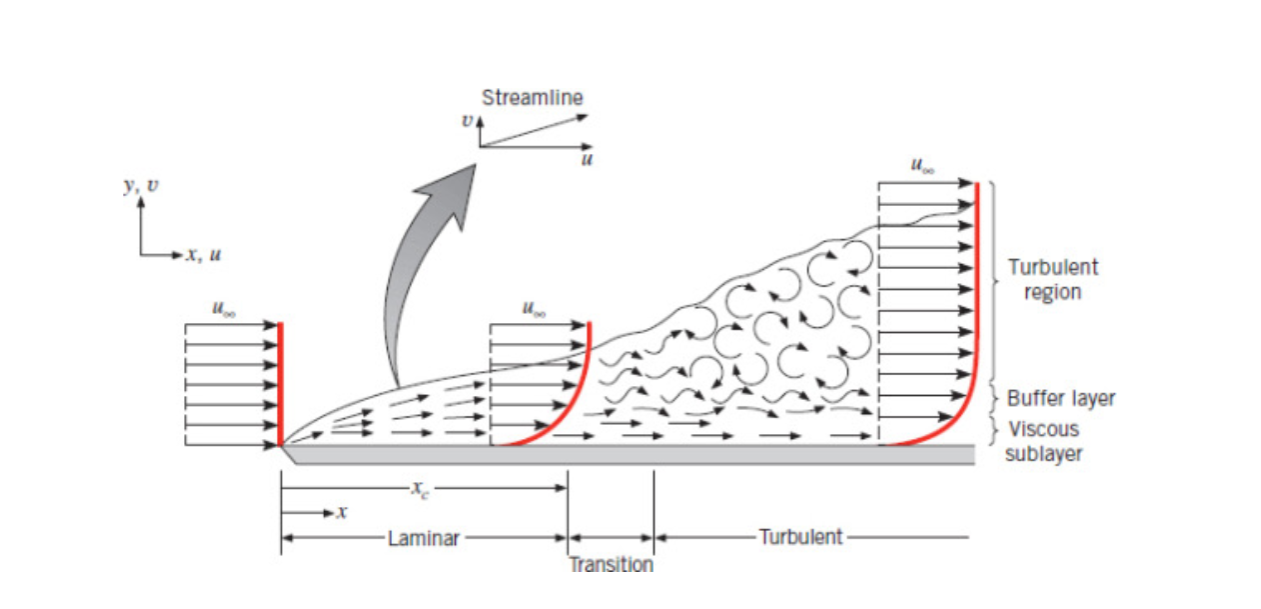
You can find the point at which flow becomes turbulent using the critical reynolds number.
The circular flow within turbulent flow are called eddies.
See ME3304 S24 Lecture Note (3) Convection for more info.
Cylinder in Cross Flow
Section 7.4
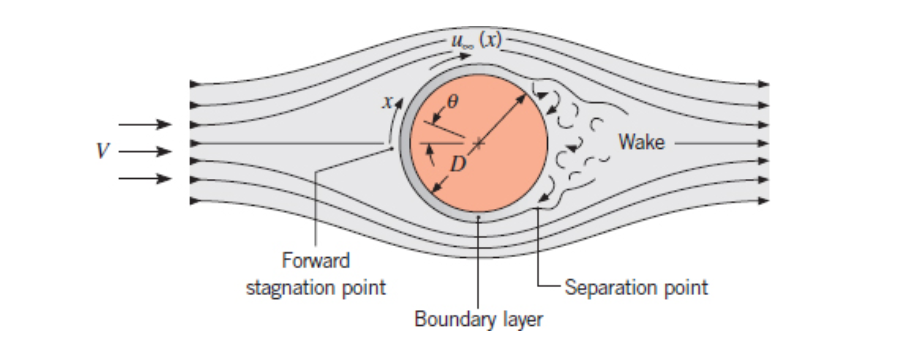
The Hilpert Correlation (Equation 7.52):
Constants
Diameter is used as the characteristic length.
External Flow
Chapter 7
Depending on your problem you can apply various models:
Flow Across Banks of Tubes
Section 7.6
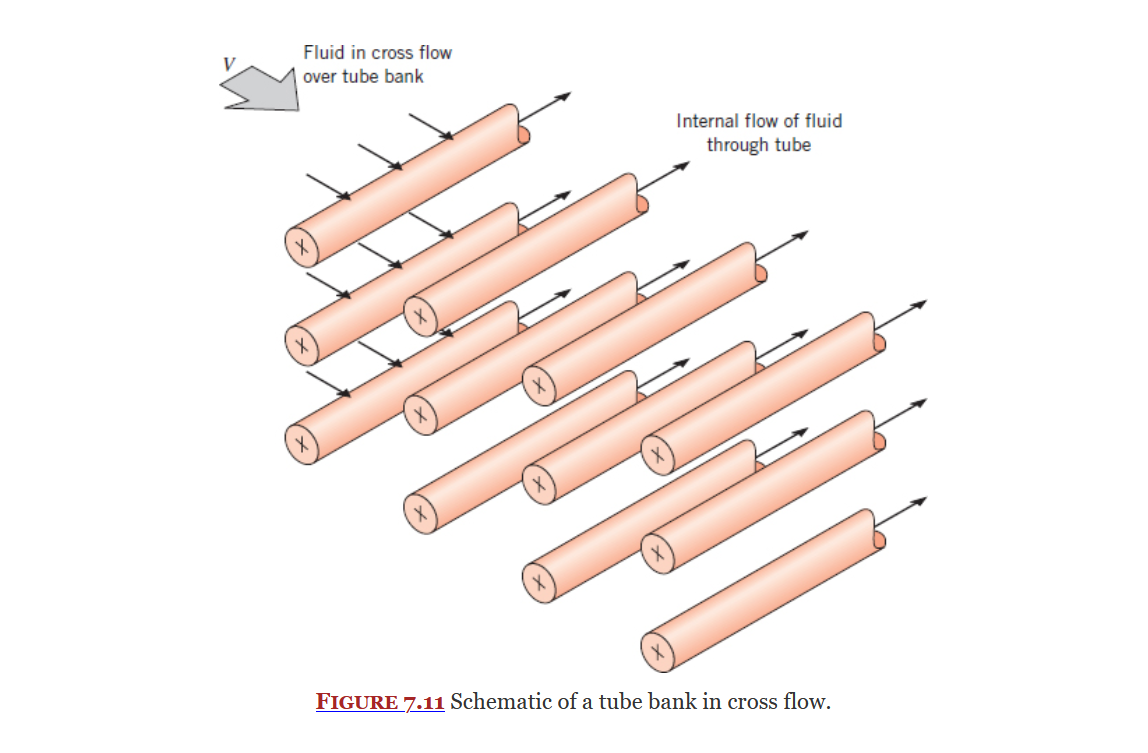
Fluid Flow
Fluid flow has a large impact on convection and needs to be accounted for when finding the convection heat transfer coefficient.
The largest two factors that effect convection are:
- Where does the flow occur? (externally or internally)
- What form does the flow take? (laminar or turbulent)
This can be found out by calculating the reynolds-number and comparing it to the typical critical-reynolds-number.
Flow might be both laminar and turbulent, boundary layers are used to analyze these flows against surfaces. There are three types of boundary layers that are of interest to this class:
- Velocity boundary layer measures fluid velocity.
- Thermal boundary layer measures temperature.
- Concentration boundary layer measures mass.
Fluid Flow makes heavy use of dimensionless parameters.
The Reynolds Analogy is used often to relate parameters in a flow.
Extended Surfaces (Fins)
Extended Surfaces or Fins are sections of an object that extend out into a fluid used for heat dissipation. They are covered in Section 3.6.
General form of energy dissipation equation for a fin (Eq. 3.66):
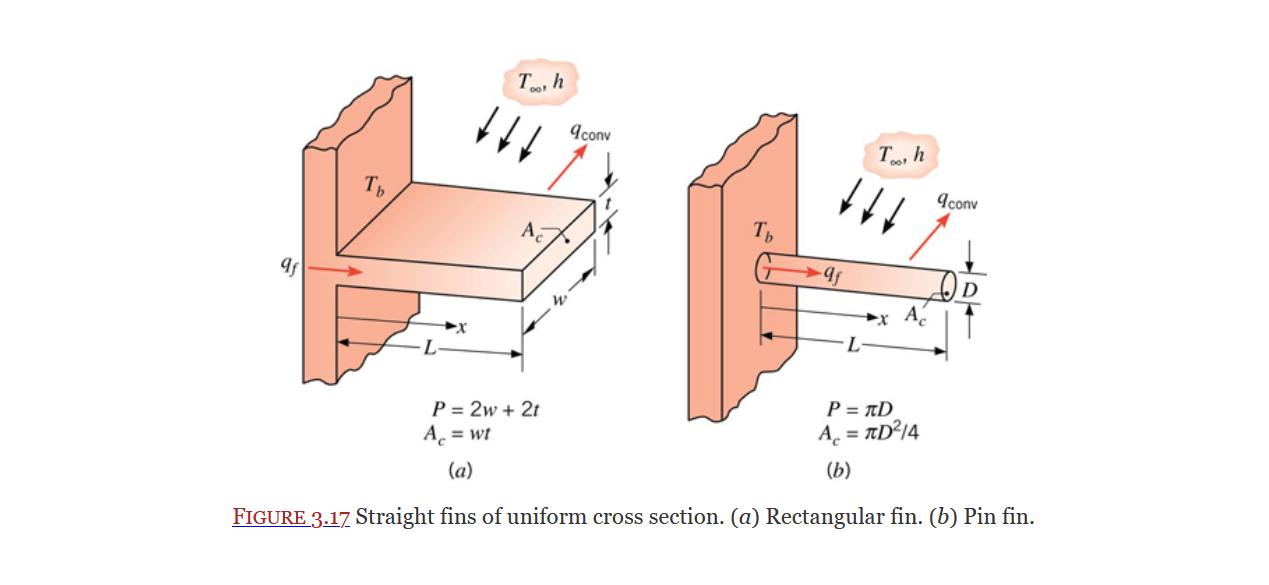
In the above image the cross section does not change along the length of the fin, thus the general equation can be reduced to the following (Eq. 3.67):
The following two definitions (Eq. 3.68 & 3.70):
allow us to reduce our equation (Eq. 3.69):
Now that it is in this form we can find it's general solution to be (Eq. 3.71):
We generally refer to
A derivation for a rod fin can be found in 4E - Class 2 - Fins Cont. 2024-02-13 00_10_36
Hyperbolic tribometry functions (namely
See extended-surface-equation-table for equations related to extended surfaces.
Fin Effectiveness
Fin Efficiency
Where
About this chart
Layout
Concepts build as they go down the chart, all ideas aim to build on the three main heat transfer methods. Arrows point from prerequisite information to the concepts they support.
Color Code
- White - General Information/Concepts and Topics
- Gray/Black - Supporting Concepts, Equations, and Ideas
- Red - conduction
- Orange - convection
- Yellow - radiation
- Green - mass transfer
- Purple - topics that contain a combination of the above three heat transfer modes
- Blue - topics related to fluid flow
Boundary Layer
A boundary is a section of fluid that flows up-against some surface. Typically the velocity is slower closer to the surface, while at some distance away from the surface
- Velocity boundary layer as mentioned above.
- Thermal boundary layer which is concerned with temperature.
- Concentration boundary layer which is concerned with mass.
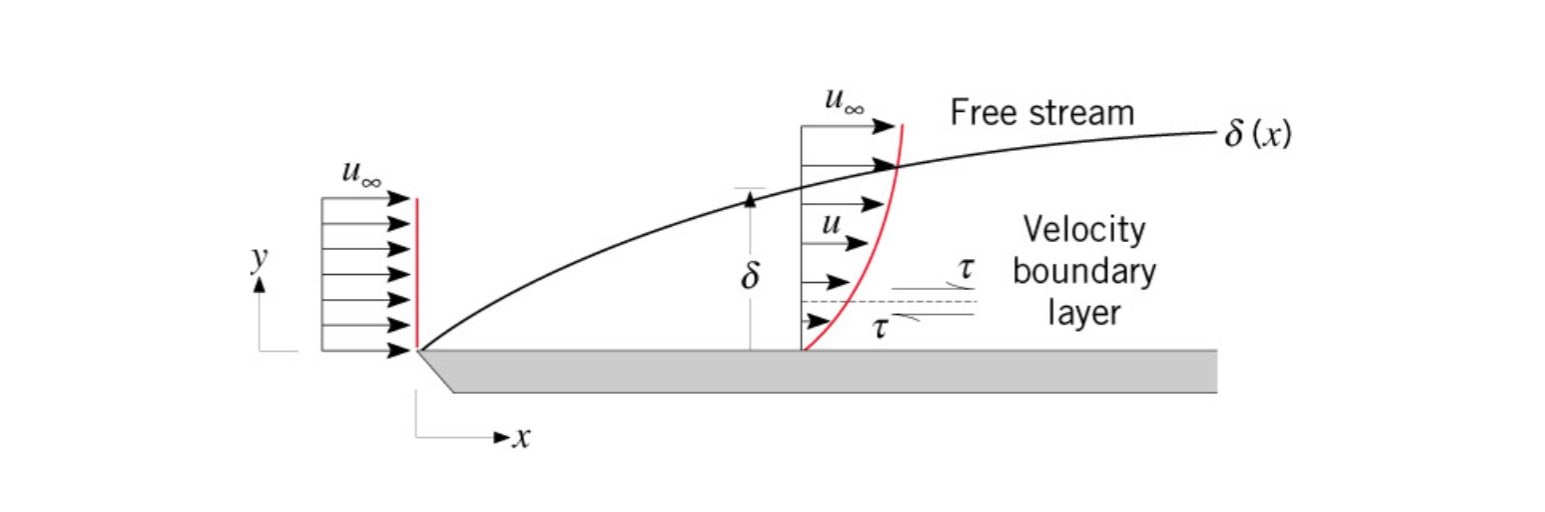
Boundary layer thickness is denoted as
There are multiple types of boundary layers, namely velocity boundary layer and thermal boundary layer.
Fluid flow inside a boundary layer can be laminar or turbulent.
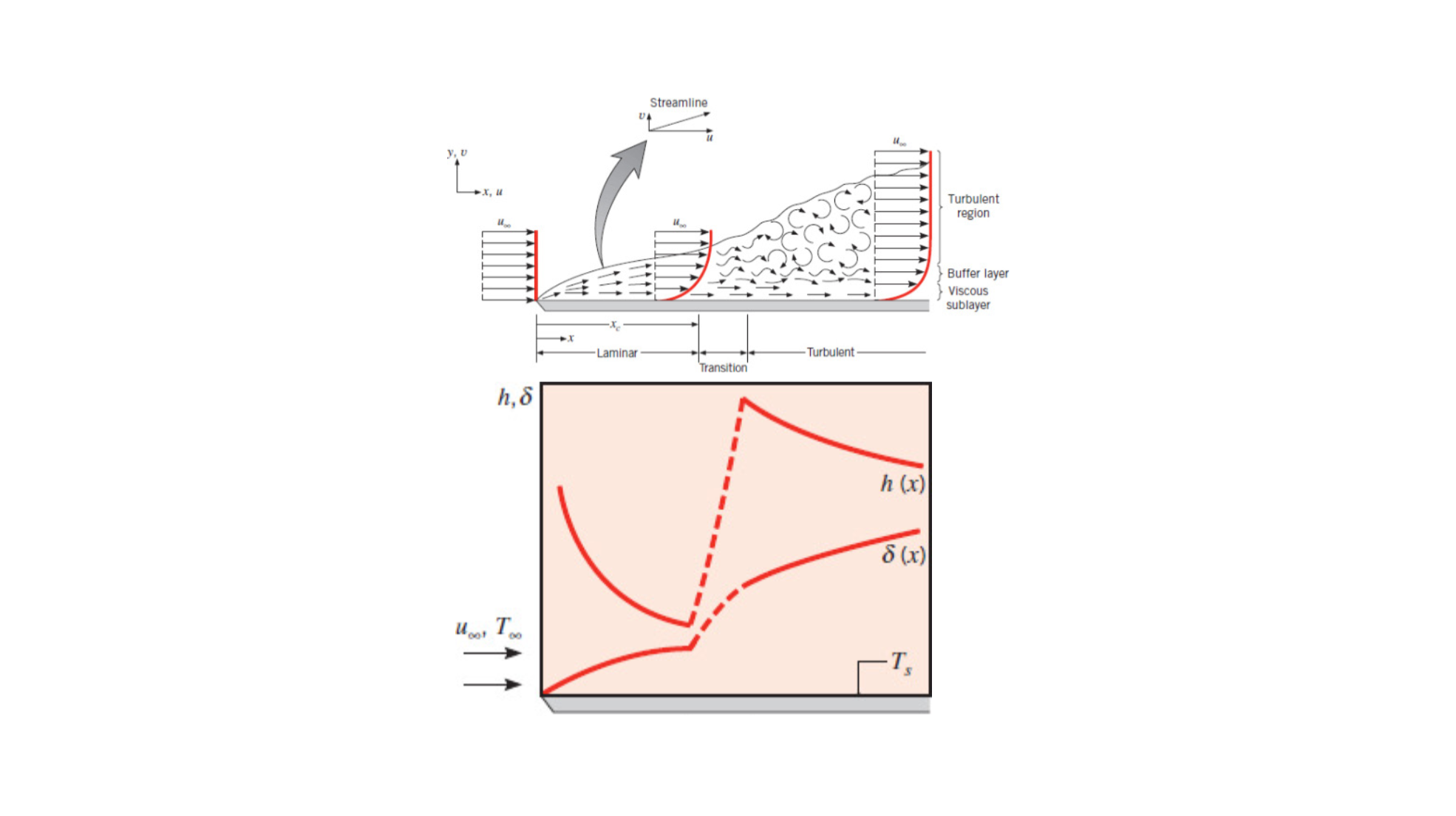
See ME3304 S24 Lecture Note (3) Convection for more information.
Heat Exchanger - Overall Heat Transfer Coefficient
Section 11.2
When this is large you have a lot of heat transfer.
- Dr. Paul
Equation 11.1a:
Where
Equation 11.1b:
Where
Heat Exchanger Effectiveness-NTU Method
Section 11.4
The Effectiveness-NTU method is an heat exchanger analysis method like the log-mean temperature analysis method but doesn't require knowing the outlet temperatures.
NTU stands for number of mass transfer units.
After defining heat exchanger effectiveness (
Equation 11.20 (Hot fluid):
Equation 11.21 (Cold fluid):
Where subscripts
Table 11.3 and Table 11.4 - Heat Exchanger NTU relations provide relationships between effectiveness
Overall Heat Transfer Coefficient
It's "overall" because it includes more than just convection.
- Dr. Paul
The overall heat transfer coefficient (
Where
Heat Exchanger Effectiveness
Section 11.4.1
Used in the effectiveness-NTU analysis method.
Defined in equation 11.19 as the ratio of actual heat transfer to the maximum possible heat transfer:
Number of Mass Transfer Units
NTU is a dimensionless parameter used in the effectiveness-NTU analysis method.
It is defined on page 508 of the text book, in equations 11.23 and 11.24. Equation 11.24 is listed below:
Where
As with all non-dimensional parameters we can think of this as a ratio of two things.
A heat exchanger will be better if this number is larger.
- Dr. Paul
Log Mean Temperature Difference Analysis
Section 11.3
Log Mean Temperature Difference Analysis is a heat exchanger analysis method that can be used if you know the inlet and outlet temperatures of your hot and cold fluids and one of the mass flow rates. You can then calculate the heat transfer between the fluids and the overall heat transfer coefficient.
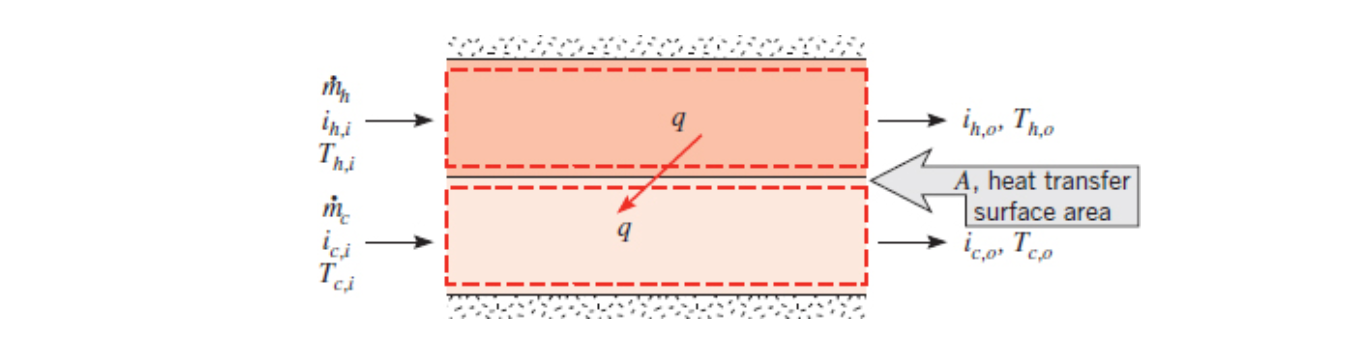
Energy balance of the hot fluid (Equation 11.6a):
Energy balance of the cold fluid (Equation 11.7a):
Where
If the fluids stay in the same phase and you can assume a constant specific heat as they change temperature then you can use temperature instead of enthalpy:
Energy balance of the hot fluid (Equation 11.6b):
Energy balance of the cold fluid (Equation 11.7b):
Where
See ME3304 S24 Lecture Note (6) Heat Exchanger and 12A - 2nd Class for more information.
See ME3304 S24 Lecture Note (6) Heat Exchanger, page 21 for information about how to determine the mean temperature.
If you need to find the heat-exchanger-overall-heat-transfer-coefficient you can use equation 11.14:
Where
Log Mean Temperature Difference is defined in equation 11.15:
For a counter flow heat exchanger they are defined by equation 11.17:
Special Operating Conditions
Section 11.3.3
Parallel-Flow Heat Exchanger
Section 11.3.1 - heat-exchangers
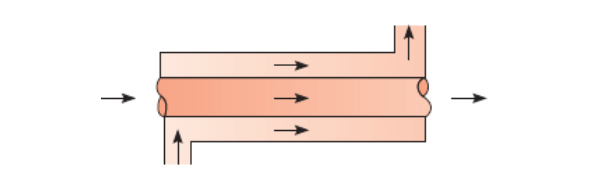
Parallel-flow is more efficient than counter-flow for shorter heat-exchangers.
Counter Flow Heat Exchanger
Section 11.3.2 - heat-exchangers
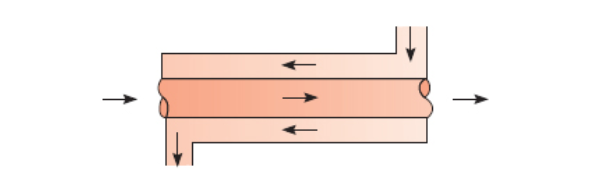
Counter-flow is more efficient than parallel-flow provided that the heat exchanger is long enough.
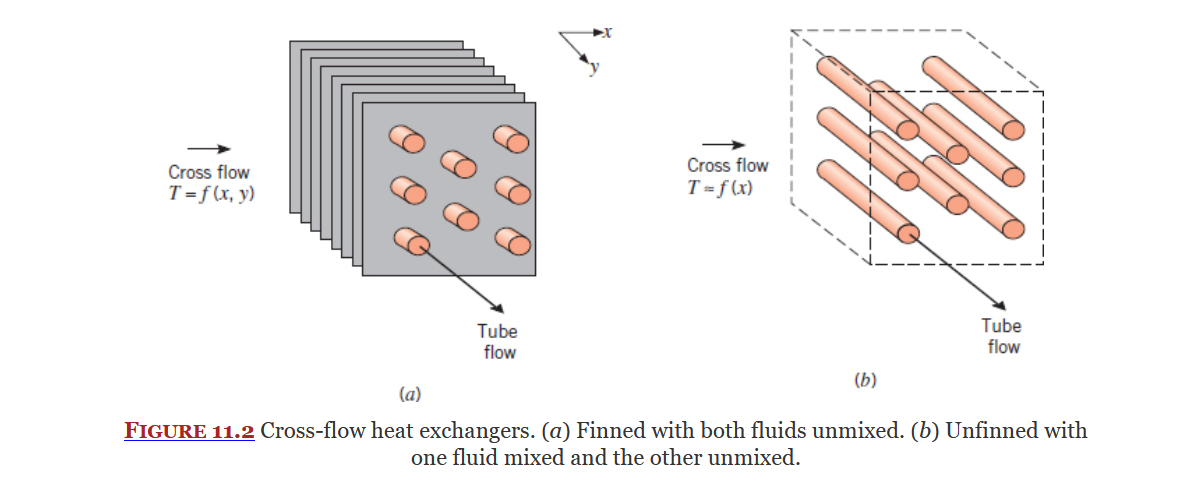
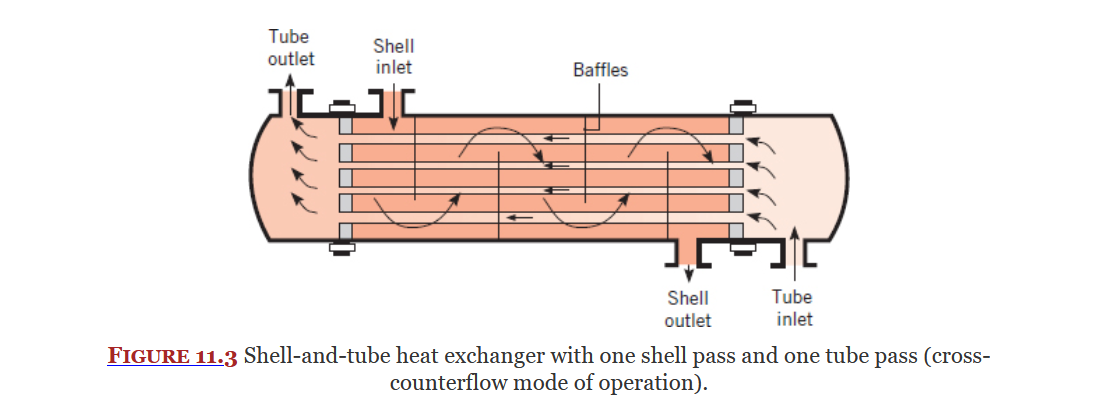
Cross Flow Heat Exchanger
Shell-Tube Heat Exchanger
Types of Heat Exchangers
Heat Capacity Rate
A measure of how much heat energy a substance than hold/take on?
Where
Defined in equations 11.10 and 11.11.
See also 12A - 2nd Class 2024-04-01 17_10_13
Analysis Methods
Heat Exchangers
Covered in Chapter 11, Heat Exchangers are used to transfer heat between two fluids.
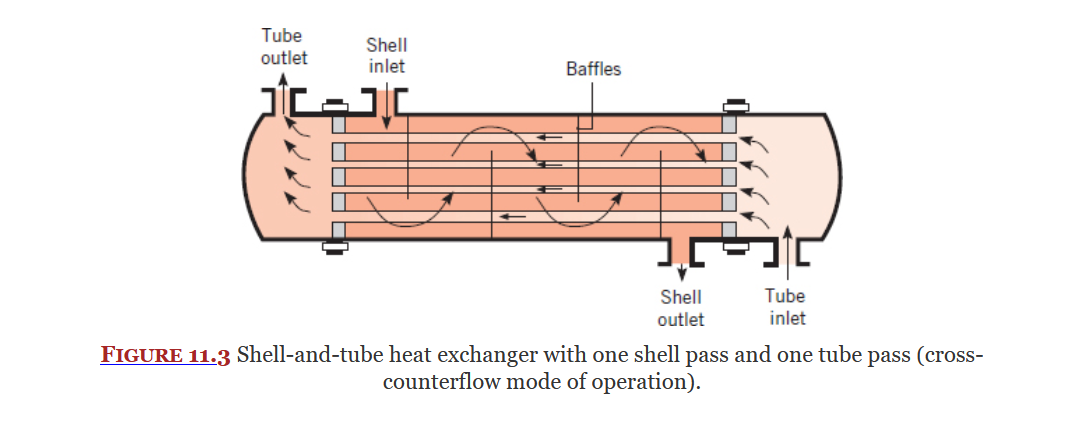
There are multiple types of heat exchangers:
Heat Transfer Analysis Methods:
- log mean temperature difference analysis - if you know the inlet and outlet temps and mass flow rate of either fluid
- effectiveness-NTU analysis method
See ME3304 S24 Lecture Note (6) Heat Exchanger for more information.
The following are notes from Dr. Paul's section covering heat exchangers:
Mass Flow Rate
Equation 8.5:
or the fluids version:
Where V is velocity and A is cross-sectional area.
Mass Transfer
Mass transfer is driven by concentration (
Species is a term commonly used to refer to some group of mass, often the group is of the same element or chemical.
A species molecular weight (
Grey Surface
Covered in Section 12.8, a grey surface is any surface that is not a blackbody, in other words, its emissivity is less than 1.
For a grey body the emissivity is equal to the absorptivity, that is:
A grey surface is a closer approximation to real surfaces but the due to the above correlation it doesn't always match real surfaces.
Reflectivity
Absorptivity
Equation 1.6, the amount of irradiation absorbed.
See also ME3304 S24 Lecture Note (7) Radiation, Page 4 and Page 15
Equation 12.47:
Transmissivity
The fraction of irradiation transmitted through the medium.
See also ME3304 S24 Lecture Note (7) Radiation.
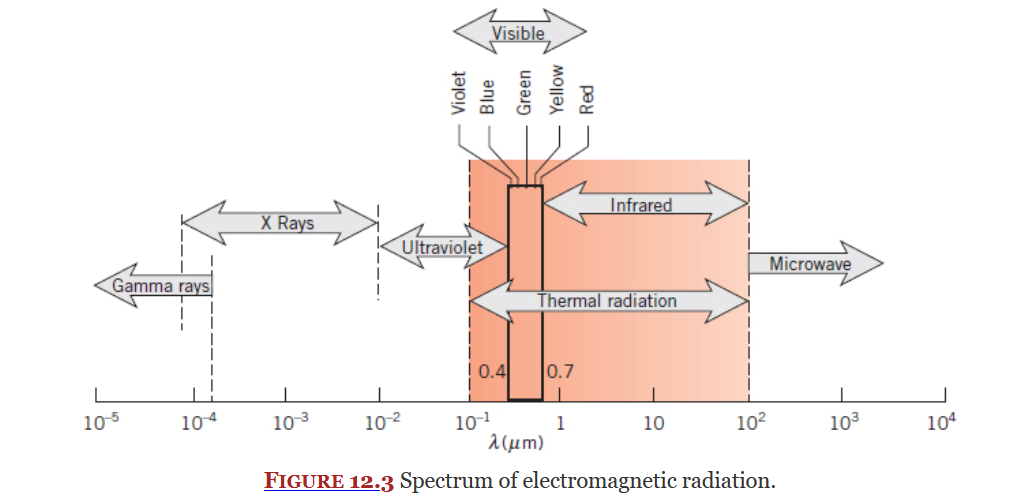
Thermal Radiation
Thermal radiation is a section of the electromagnetic radiation spectrum. It incudes some of ultraviolet, all of visible light, and all of infrared. Thermal radiation is an electromagnetic wave.
See ME3304 S24 Lecture Note (7) Radiation and Section 12.1 for more information.
Radiation Heat Fluxes
Covered in Section 12.2, and listed out in table 12.1, the four radiation heat fluxes are the components that make up the energy transfer by way of radiation.
These components are indeed heat fluxes and as such have units of
- emissive power,
- irradiation,
- radiosity,
- net radiative flux,
See ME3304 S24 Lecture Note (7) Radiation for more info.
Emissive Power
Emissive Power is a radiation heat flux and is represented by
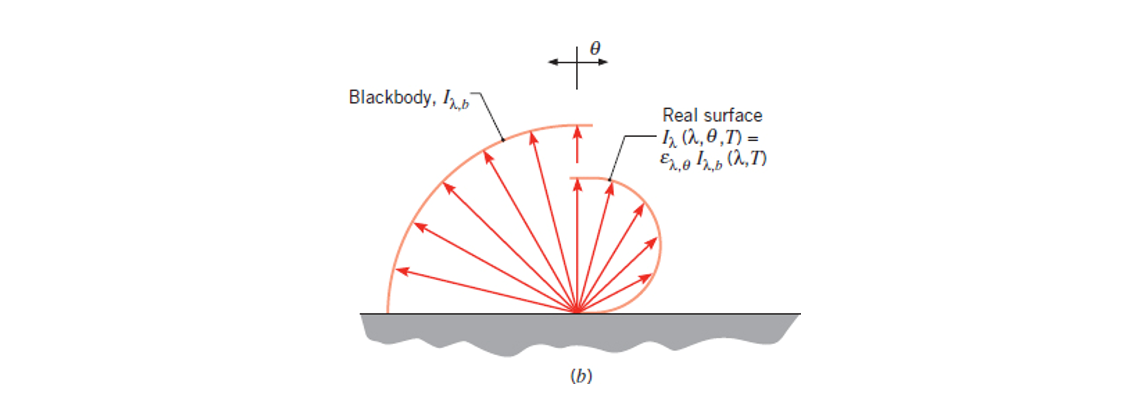
If your object is an ideal radiator it is called a black body, and its radiation
Otherwise, if your object is a real surface its radiation is instead given by Equation 1.5:
Where
Spectral Emissive Power
Equation 12.15:
Where
See 14C - 2nd Class for more information.
For a diffuse emitter:
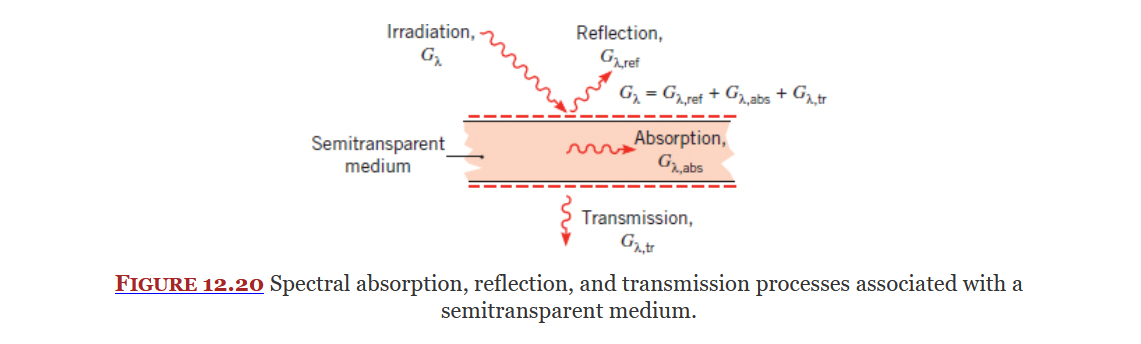
Irradiation
Covered in Section 12.2, Irradiation is the radiation incident (acted upon) on a surface. It is a radiation heat flux represented by
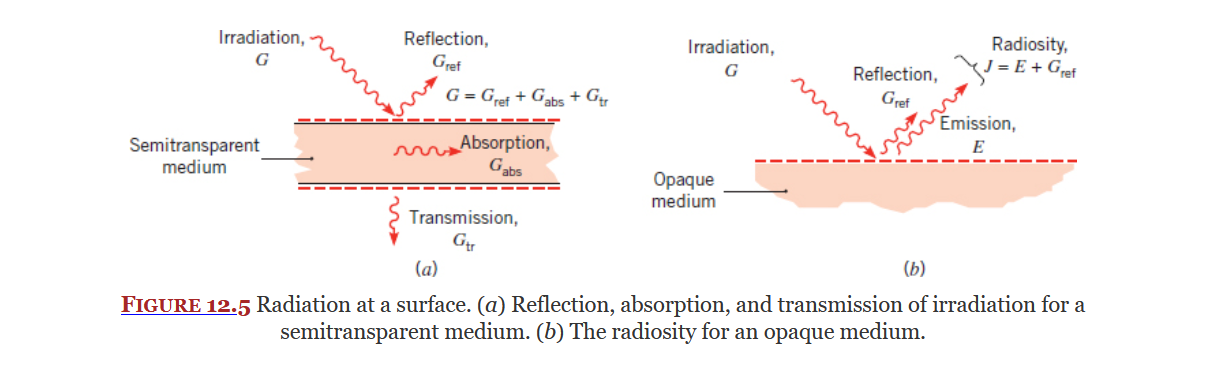
See ME3304 S24 Lecture Note (7) Radiation and 14A - 2nd Class for more info.
Components
There are three main components to irradiation.
For most surfaces you can use Equation 12.2:
For an opaque medium there is no transmission so Equation 12.2 becomes 12.3:
See Section 12.2 and ME3304 S24 Lecture Note (7) Radiation for more infomation.
Spectral Irradiation
Radiosity
Radiosity is a radiation heat flux, it is represented by
For an opaque surface:
Where
Spectral Radiosity
Net Radiative Flux
Listed in table 12.1 as the following:
Where
Blackbody
Covered in section 12.4, a black body is an object/solid that absorbers all radiation it receives, emits perfectly (it has an emissivity of 1), and emits in all directions (diffuse emitter).
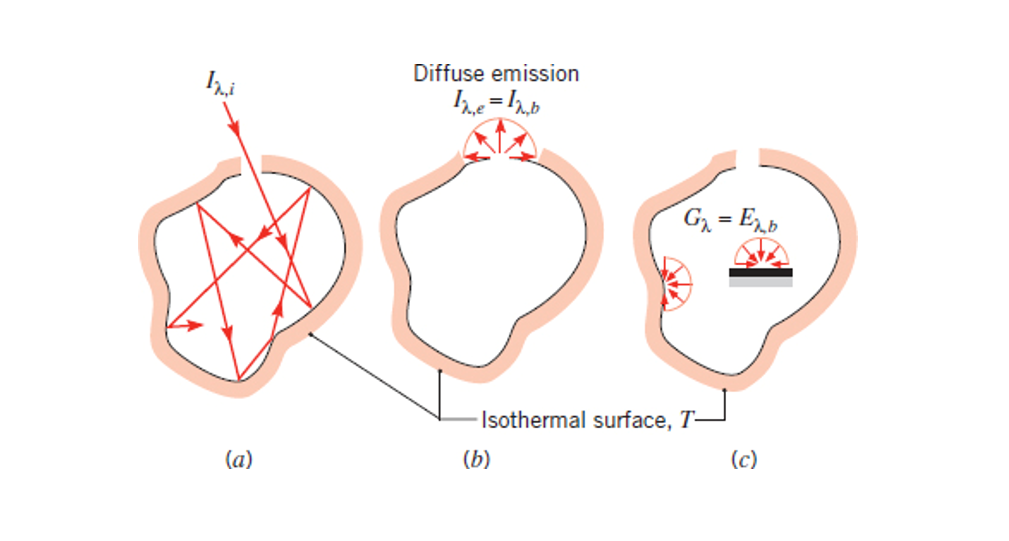
Blackbodies are:
- Perfect absorbers (
- Perfect emitters (
- Grey - radiation does not depend on (
- Emitted radiation is a function of wavelength (
See ME3304 S24 Lecture Note (7) Radiation and 14E - 2nd Class for more information.
Equation 12.29 (blackbody spectral intensity):
See 14E - 2nd Class for details about this equation.
A comparison of a black body to a real surface can be found on Slide 32 of ME3304 S24 Lecture Note (7) Radiation.
Emissivity
A material property, defined in Section 12.5, it measures how efficiently a surface emits energy in reference to a black body.
In other words it is the ratio of the radiation emitted by a surface to the radiation emitted by a blackbody of the same temperature.
Used in Equation 1.5 and Example 5.2.
Equation 12.38:
Where
Radiation Intensity
Section 12.3
radiation + geometryyyyy
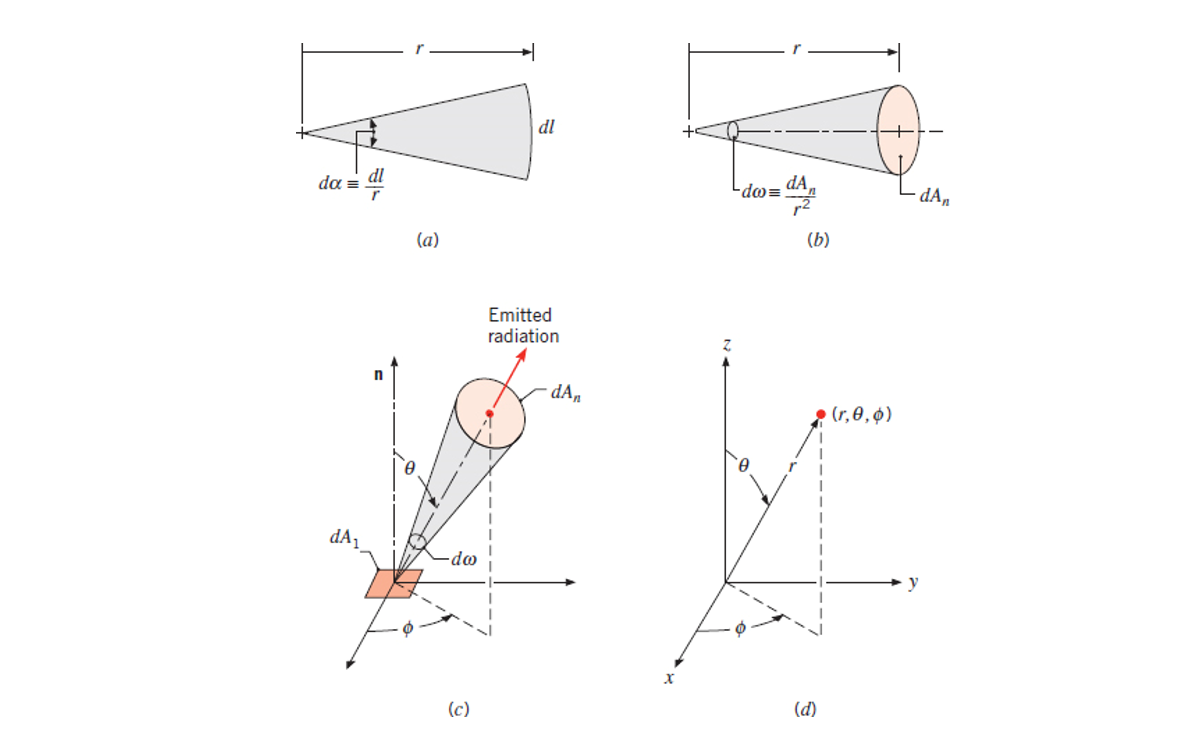
Figure 12.6 - Mathematical definitions (a) Plane angle. (b) Solid angle. (c) Emission of radiation from a differential area
into a solid angle subtended by at a point on . (d) The spherical coordinate system.
Solid Angle
Covered in Section 12.3.1, the solid angle (
Equation 12.9:
Where
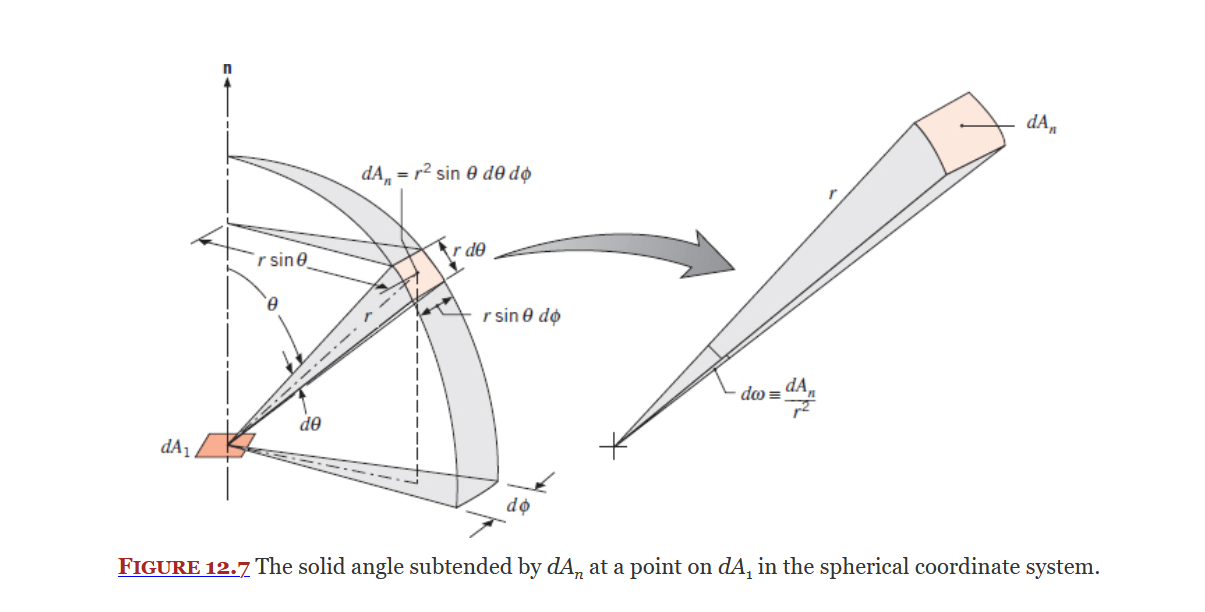
The unit of the solid angle is steradian (sr), equal to radians for plane angles?
Spectral Intensity
Defined in Section 12.3.2 by Equation 12.10:
Where
Radiation
Covered in Chapter 12 and 13, radiation is heat transfer due to changes in election configuration, it takes the form of electromagnetic waves.
Radiation exists on electromagnetic radiation spectrum (Figure 12.3 below), a subset of this spectrum is thermal radiation which is what this course focuses on. Thermal radiation is an electromagnetic wave.
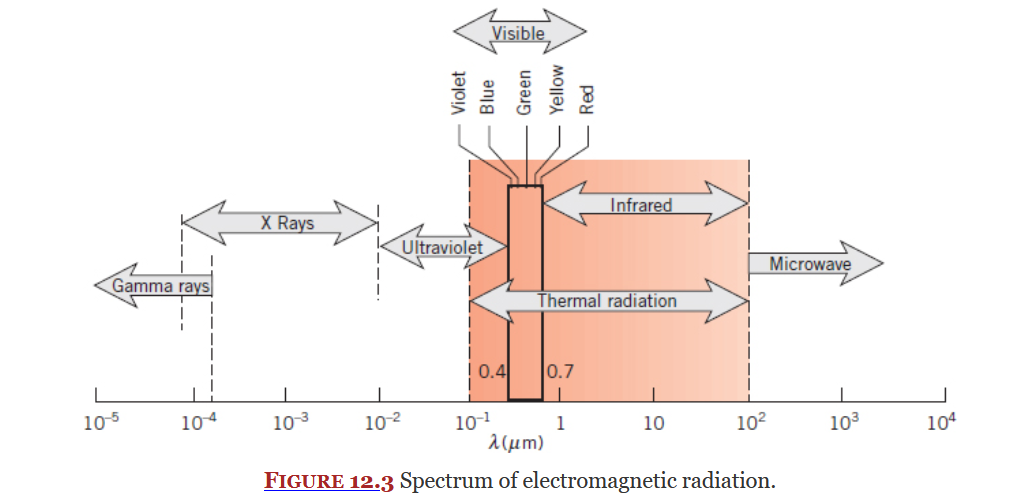
From 13E - 2nd Class:
Thermal radiation is measured via four different radiation heat fluxes defined in table 12.1:
See ME3304 S24 Lecture Note (7) Radiation for more info.
When working with radiation, Dr. Paul recommends to always convert to Kelvin.
Radiation Balance
Opaque Medium
An opaque medium just means there is no radiation that makes it all the way though.
- Dr. Paul
Where
View Factor
Covered in Section 13.1, the view factor is defined by the book as the fraction of radiation leaving surface i that is intercepted by surface j.
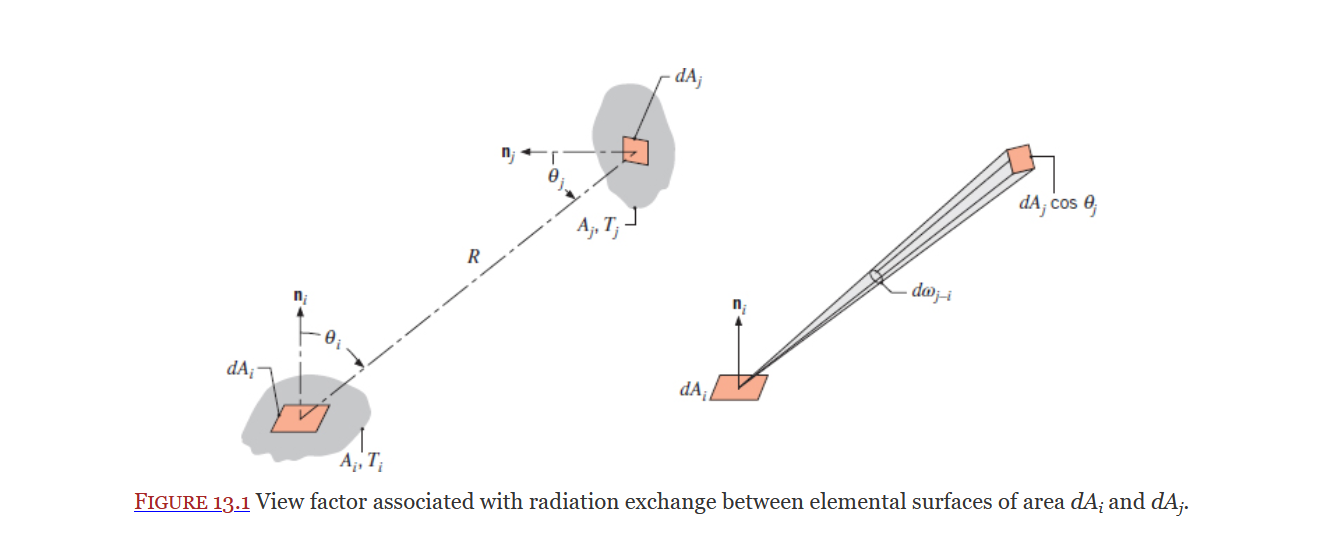
View factors for 2D geometries can be found on table-13.1.
View factors for 3D geometries can be found on table-13.2.
Rules / Relations
Reciprocity Relation (Eq. 13.3):
Summation Rule (Eq. 13.4) for surfaces in an enclosure:
Where
See ME3304 S24 Lecture Note (7) Radiation and 15A - 2nd Class for more information.
Fick's Law
Covered in Section 14.1.3, Fick's Law is like Fourier's law but applied to mass transfer.
Equation 14.12:
Where
Equation 14.13:
Where
See ME3304 S24 Lecture Note (8) Mass Transfer for more information.
Mass Diffusion
Covered in Chapter 14, mass diffusion is the diffusion (or movement) of mass from a high concentration to an area low concentration.
Where
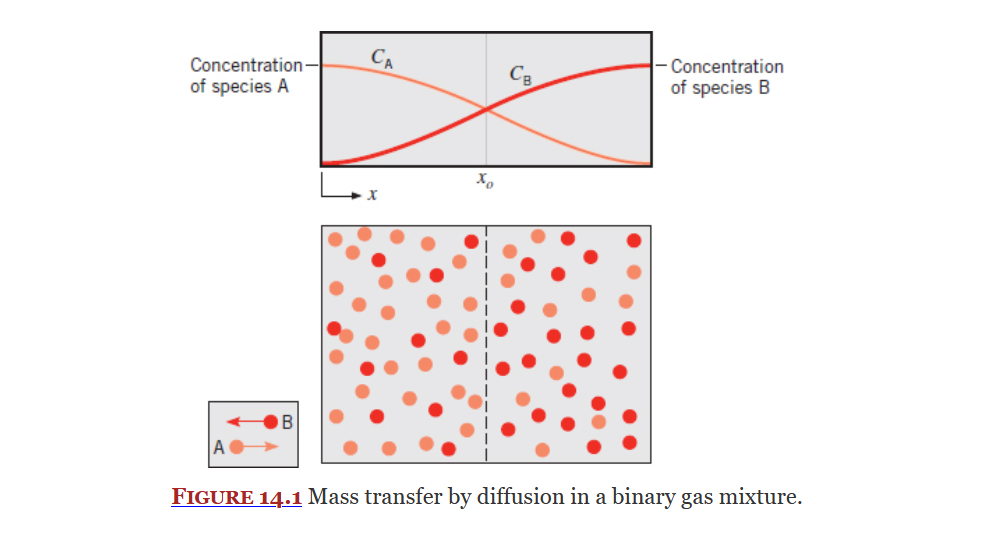
Equation 14.14 (Slide 8 of ME3304 S24 Lecture Note (8) Mass Transfer):
Where
See ME3304 S24 Lecture Note (8) Mass Transfer for more information.